PCSP Technical Advisory No. 004
Series of 2020
RECOVERY FROM ASF
SAMPLES & TESTS FOR ASF DETECTION
A key to ASF control is early detection. Routine diagnostic tests are essential to identify sick from healthy animals to ensure that only healthy animals are harvested.
If a farm observes feverish animals, inappetence, difficulty in movement (lethargic), with or without the typical skin lesions, and an unusual increase in daily mortality trend within the last 7 days, ASF must be suspected as one of the differential diagnosis.To guide the swine farmer and veterinarian on the submission of the correct samples and the use of tests, the PCSP prescribes the following:
Samples
Types of Samples
Primarily whole blood, from which serum (coagulated) or plasma (with anticoagulant) can be collected, depending on the test to be done.
Alternatively, tissue samples from organs (spleen) from dead animals can be collected as a last resort. This is strongly discouraged since this will require opening the animal, and cause blood spillage. Blood contains vast amount of virus that could contaminate the area.
Keep samples in a cold container and submit to the laboratory within 24 hours.
The laboratory may do pooling of five (1:5) samples or more (1:10, 1:20) to maximize resources.
Condition of pigs where samples should be taken:
- Recently dead > Acutely Sick > Healthy;
- Prioritize suspect animals (dead and sick). Collect at least 10 from morbid animals.
- Only collect from healthy animals if the sample size is not reached.
- The aim is to detect the infection at the right time to prevent further transmission.
Sample size
Number of samples is dependent on the farm size, and for most farms thirty (30) samples is sufficient. Refer to your veterinarian for the correct sample size.
Frequency of sampling collection is dependent on purpose:
- For movement of pigs, refer to BAI guidance for certifications;
- For in-farm decision making, such as in sentinel programs, as often as necessary; and
- For surveillance purposes, refer to your LGU veterinarians.
Only send samples to government and BAI-accredited diagnostic laboratories to ensure credibility of results. Please consult with your veterinarian for more details.
Tests
The use of diagnostic tests to detect ASFV should be based on the understanding of the Dynamics of ASFV Infection and the scope and limitation of each test.
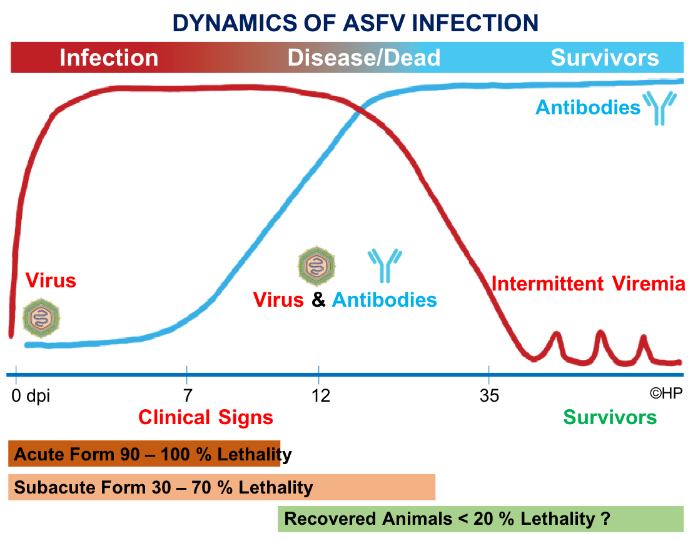
The figure summarizes the emergence of ASF virus in blood and the subsequent production of antibodies upon ASFV infection. In addition, it also shows the lethality of the different forms of the clinical disease. Heat chart denotes infection and presence of Virus (red) that transitions to probable survivors and production of antibodies (light blue). Figure adapted and modified by Dr. Homer Pantua, from Gallardo, MC et al. 2015. Pig Health Management; Alcrudo, DB, Gallardo MC, Arias M, Penrith ML. 2017. African Swine Fever: Detection and Diagnosis.
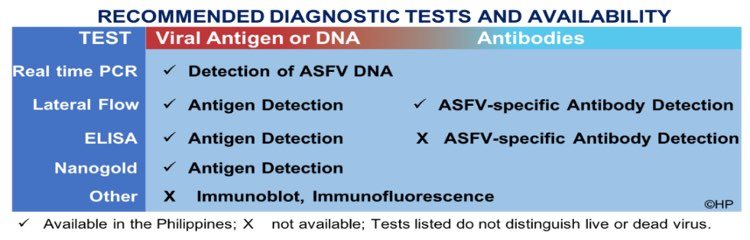
Diagnostic tests to be used should be based on the stage of infection as determined by the veterinarian and farmer.
Detection of genetic material and antigen, which is recommended in the early stages of infection, will show the presence of virus, suggestive of an active infection. In contrast, detection of antibody at the later stages of infection suggests a response to exposure to the virus. Diagnostic tests could either be performed in the laboratory (PCR, ELISA) or at the point of need (LFD, Portable PCR, Nanogold).
It is best to consult with a veterinarian and laboratory diagnostician for advice on the most appropriate tests with sensitivity, specificity as well as economic considerations.
A positive or negative result from the tests listed in the table only suggest the potential presence or absence of virus and/or its genetic material. To determine the presence of infectious virus, laboratory tests such as isolation of the virus could be performed. A comprehensive analysis of both clinical and laboratory findings is important to generate a well guided diagnosis.
Certifications
The laboratory PCR test results are important to comply with the BAI issued memos and the National Zoning and Movement Plan for ASF document. The other laboratory tests may be used in farm diagnosis and programs.
A negative result for all samples submitted by the farm would mean the issuance of the Certificate of Farm Disease Free status – ASF, the validity of which ranges from 7 to 21 days depending on BAI and LGU guidelines.
We hope the veterinarian and swine farmer will be guided so we can detect our ASF cases faster and control its spread.

 I
I

Leave A Comment